ITEM SPECIFICS
-
Brand
Model M251drop
-
origin
Republic of Korea
-
Size(Capacity)
96 Test
-
Function
Simultaneous diagnosis of COVID-19 and a viral respiratory infection
-
Gender
All gender
-
age-appropriate
Adult, Senior & Disabled Person
-
Expiry Date
1 year from date of manufacture
-
Condition
Freezing
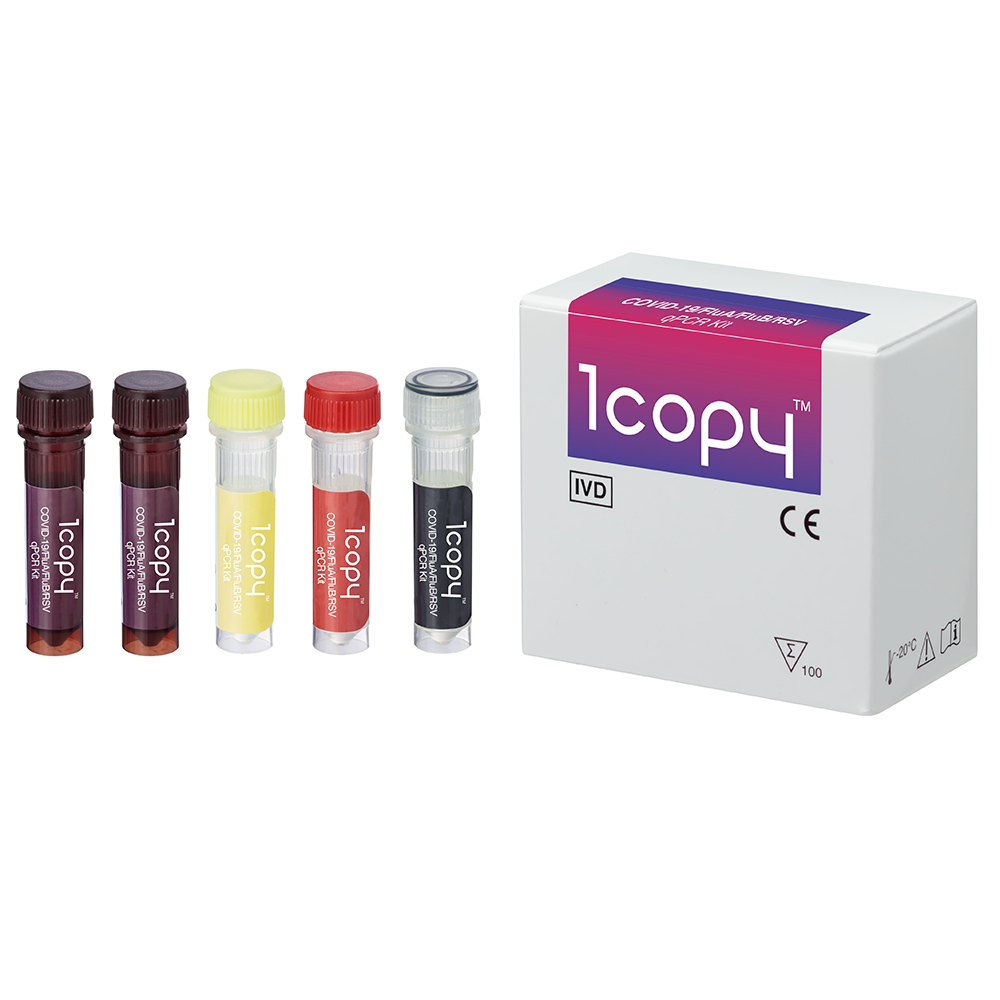
PRODUCT DESCRIPTION
COVID-19/InfluenzaA/InfluenzaB/RSV qPCR Kit(PCR)
Intended Use
1copy™ COVID-19/FluA/FluB/RSV qPCR Kit is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from SARS-CoV-2, Influenza A virus(FluA), Influenza B virus(FluB) and Respiratory Syncytial Virus (RSV) in nasopharyngeal, oropharyngeal, anterior nasal, mid-turbinate nasal swab specimens as well as nasopharyngeal wash/aspirate and nasal aspirate specimens collected from individuals suspected of COVID-19 by their healthcare provider.
Advantages
1. Honest price through direct production/distribution.
2. Simultaneous diagnosis of COVID-19 and a viral respiratory infection.
2. Simultaneous diagnosis of COVID-19 and a viral respiratory infection.
How to use
1. Mixture Preparation
2. Sample Preparation
3. Enter settings for the 1copy™ COVID-19/FluA/FluB/RSV qPCR Kit on each PCR instrument and software
4. Start Test
5. Checking the result.
2. Sample Preparation
3. Enter settings for the 1copy™ COVID-19/FluA/FluB/RSV qPCR Kit on each PCR instrument and software
4. Start Test
5. Checking the result.
Cautions
1. This product is intended for in vitro diagnostic use only.
2. prevent contamination.
2. prevent contamination.
Company Overview
We are a fast growing healthcare company that provides innovative solutions for the prevention, improvement, and monitoring of disease.
We will lead the innovations and changes among the global healthcare industry in the field of mobile healthcare, a digital platform where you can measure various components of the blood with a single smartphone and biosensor, and in the field of molecular diagnostics, a form of accurate and stable molecular detection of biomarkers with 1drop's special technology.
We will lead the innovations and changes among the global healthcare industry in the field of mobile healthcare, a digital platform where you can measure various components of the blood with a single smartphone and biosensor, and in the field of molecular diagnostics, a form of accurate and stable molecular detection of biomarkers with 1drop's special technology.
Other Products
R&D CERTIFICATE
PAYMENTS DETAILS
This supplier supports payments for offline orders
- Telegraphic Transfer : T/T
- Name : Wonjung Kim
SHIPPING
Shipping from :
Republic of Korea
- 215 Galmachi-ro Jungwon-gu, Seongnam-si, Gyeonggi-do (13217)
1drop Inc.
The person in charge
Joowon RheeAddress
215 Galmachi-ro Jungwon-gu, Seongnam-si, Gyeonggi-do (13217)
Introduction
We are a fast growing healthcare company that provides innovative solutions for the prevention, improvement, and monitoring of disease.
We will lead the innovations and changes among the global healthcare industry in the field of mobile healthcare, a digital platform where you can measure various components of the blood with a single smartphone and biosensor, and in the field of molecular diagnostics, a form of accurate and stable molecular detection of biomarkers with 1drop's special technology.
∙ Factory Information
1drop Inc (www.1drop.co.kr)
Address : A-203, 1001, 1002, Keumkang Penterium IT Tower, 215, Galmachi-ro, Jungwon-gu, Seongnam-si, Gyeonggi-do, 13217, Republic of Korea
E-mail : sales@1drop.co.kr Tel : 031-747-0109
∙ Business Overview
1) Point-of-Care Molecular Diagnostic Platform Consisted of Reagent + Device + App
2) 1drop™, Smartphone-based Solution for Monitoring of Chronic Disease
3) 1copy™, qPCR Kit Using Widely Distributed PCR Machines
∙ Vision
We aim to contribute to humanity by providing world-class healthcare products and services with our team of experts and unique technology.
-
- Business Type :
- Manufacturer
-
- Main Product :
- 1drop™, 1copy™
-
- Established :
- 2017-09-25
-
- Total Annual Revenue :
- 6 million to 10 billion (KRW)
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
-
- CE (IVDR_Class_A)1POT_Duo_PCMAP
- MDSS
- 2023.08.17
- 인증서보기
-
- CE C18_DOC_1drop™ Total Cholesterol
- MT Promedt Consulting GmbH
- 2019.07.12
- 인증서보기
-
- CE G18_DOC_1drop™ Glucose
- MT Promedt Consulting GmbH
- 2019.06.28
- 인증서보기
-
- CE H18_DOC_1drop™ Hemoglobin
- MT Promedt Consulting GmbH
- 2019.01.04
- 인증서보기
-
- CE U18_DOC_1drop™ Uric Acid_v1.0
- MT Promedt Consulting GmbH
- 2019.06.05
- 인증서보기
-
- KMFDS 1POT_Duo_PCMAP
- NIDS
- 2023.08.22
- 인증서보기
-
- KMFDS 1drop_Total_Cholesterol_(Professional)
- NIDS
- 2023.11.30
- 인증서보기
-
- KMFDS 1drop_Glucose_(Professional)
- NIDS
- 2023.11.30
- 인증서보기
-
- KMFDS 1drop_Hemoglobin_(Professional)
- NIDS
- 2023.11.29
- 인증서보기
-
- KMFDS 1drop_Uric_Acid_(Professional)
- NIDS
- 2023.11.30
- 인증서보기
-
- KMFDS 1drop_Creatinine_(Professional)
- NIDS
- 2024.02.28
- 인증서보기
-
- KMFDS
- Ministry of Food and Drug Safety
- 2021.02.24
- 인증서보기
-
- CE
- MT Promedt Consulting GmbH
- 2019.04.16
- 인증서보기
-
- KMFDS
- Ministry of Food and Drug Safety
- 2020.04.03
- 인증서보기
-
- CE
- MT Promedt Consulting GmbH
- 2021.02.05
- 인증서보기
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 6 million to 10 billion (KRW)
-
- Total export revenue (previous year in USD)
- 362
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- 1drop Inc. Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★