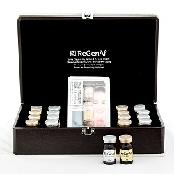
ITEM SPECIFICS
-
Brand
NYAAM NYAAM
-
origin
Republic of Korea
-
Size(Capacity)
18.5*20.5*3.5 (cm)
-
Weight
340g
-
Function
Whitening, Regeneration, Elasticity
PRODUCT DESCRIPTION
* Composition: Nyaam Nyaam device with solution: 1ea
Additional Serum : 3 pcs
ReGenAf EGF & Gold Hydro-Gel Mask Pack: 4 sheets
Additional Serum : 3 pcs
ReGenAf EGF & Gold Hydro-Gel Mask Pack: 4 sheets
* Needle: Thinner than a human so No Pain, No Blood,
Spiral Groove & Gold plated, 0.25mm of needle length
* Functional Cosmetics for Whitening
■ Vita Serum : Pigmentation, Blemish cares, Skin Vitality, Skin Tone-Up.
■ Peptide Serum : Botox effect, Elasticity, Anti-wrinkle, Anti-aging.
■ Skin Reborn Serum : Regeneration, Healing of damaged & troubled skin, Anti-aging.
■ Mulberry Stem Serum : Water Bomb, Moisturizing, Rejuvenation, Anti-aging.
* Hydro Gel Mask Pack
- 4pcs of mask sheets per pack (box)
- 100% of Pure Hydro-Gel Mask Sheet so it fits close to faceline perfectly.
- Containing EGF for Anti-wrinkle, Anti-aging, Cell regeneration.
- Containing Gold for Brightening
- For Soothing, Elasticity, Nutrition, Moisturizing
- Dermatologist tested & CFDA registeration
- The mask sheet is melted into hot water so after using it you can reuse it with essence for face and body!
Spiral Groove & Gold plated, 0.25mm of needle length
* Functional Cosmetics for Whitening
■ Vita Serum : Pigmentation, Blemish cares, Skin Vitality, Skin Tone-Up.
■ Peptide Serum : Botox effect, Elasticity, Anti-wrinkle, Anti-aging.
■ Skin Reborn Serum : Regeneration, Healing of damaged & troubled skin, Anti-aging.
■ Mulberry Stem Serum : Water Bomb, Moisturizing, Rejuvenation, Anti-aging.
* Hydro Gel Mask Pack
- 4pcs of mask sheets per pack (box)
- 100% of Pure Hydro-Gel Mask Sheet so it fits close to faceline perfectly.
- Containing EGF for Anti-wrinkle, Anti-aging, Cell regeneration.
- Containing Gold for Brightening
- For Soothing, Elasticity, Nutrition, Moisturizing
- Dermatologist tested & CFDA registeration
- The mask sheet is melted into hot water so after using it you can reuse it with essence for face and body!
R&D CERTIFICATE
-
- EC CERTIFICATE
- Institute for Testing and Certification, Inc.
- 2013.09.19
- 인증서보기
-
- Establishment Registration
- U.S. Food and Drug Administration
- 2018.03.12
- 인증서보기
-
- EN ISO 13485:2012
- Institute for Testing and Certification, Inc.
- 2016.04.28
- 인증서보기
-
- Certificate of Manufacturing Medical Devices
- National Institute of Medical Device Safety Inform
- 2016.02.02
- 인증서보기
-
- License of Medical Devices
- Taiwan Ministry of Health and Welfare
- 2015.12.28
- 인증서보기
PAYMENTS DETAILS
This supplier supports payments for offline orders
- West Union : WU
- Telegraphic Transfer : T/T
- Name : Sujeong Ma
SHIPPING
Shipping from :
Republic of Korea
- 149-6 Yuram-ro, Dong-gu, Daegu (41059)
U-BioMed Inc.
The person in charge
NYEON SIK EUMAddress
149-6 Yuram-ro, Dong-gu, Daegu (41059)
Introduction
U-BioMed Inc. is a Bio, Medical and Cosmetics manufacturer who has created advanced, innovative, micro needle technology from practical products.
Anticipating the demands of a continuously evolving market, U-BioMed has focused on the development of Medical devices, Functional Cosmetics and Bio-Medical supplements.
U-BioMed Inc., plans to lead clinical and aesthetic advancement in beauty and medical operations, through continuously developing diversified operating techniques.
We are proud to deliver what South Korean manufacturers are best know for premium quality and service.
Our main product line consists of: Tappy Tok-Tok, a painless non-surgical MTS delivery device that has been tested and approved with FDA, CE, ISO, KFDA certifications. We also have ReGenAf, a functional cosmetics line, NYAAM NYAAM for home care and STLONG line which is a product for hair care, and KIOM-MA 128 Scutellaria which is registered in International Cosmetics Ingredients Dictionary.
-
- Business Type :
- Manufacturer
-
- Main Product :
- Tappy Tok Tok
-
- Established :
- 2009-08-24
-
- Total Annual Revenue :
- 1~2 billion (KRW)
-
- Total Employees :
- 11~50 people
R&D CERTIFICATE
-
- EC CERTIFICATE
- Institute for Testing and Certification, Inc.
- 2013.09.19
- 인증서보기
-
- Establishment Registration
- U.S. Food and Drug Administration
- 2018.03.12
- 인증서보기
-
- EN ISO 13485:2012
- Institute for Testing and Certification, Inc.
- 2016.04.28
- 인증서보기
-
- Certificate of Manufacturing Medical Devices
- National Institute of Medical Device Safety Inform
- 2016.02.02
- 인증서보기
-
- ISO 13485
- ITC
- 2016.04.28
- 인증서보기
-
- CE
- ITC
- 2013.09.19
- 인증서보기
-
- ISO
- ITC
- 2016.04.28
- 인증서보기
-
- Establishment Registration
- U.S. Food and Drug Administration
- 2020.01.01
- 인증서보기
-
- License of Medical Devices
- Taiwan Ministry of Health and Welfare
- 2015.12.28
- 인증서보기
-
- US Patent
- The United States Patent and Trademark Office
- 2015.05.05
- 인증서보기
COMPANY ENVIRONMENT
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Captcha Required
Please enter the text on the left image to prevent automatic input.
0 / 4000
질문이 없습니다.
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 1~2 billion (KRW)
-
- Total export revenue (previous year in USD)
- 3
-
- Number of foreign trade employees
- 11~50 people
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
-
 PLATINUM
PLATINUM
- U-BioMed Inc. Seller's Store
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★